RPR + Automation
The RPR (Rapid Plasma Reagin) test is a nontreponemal assay for detecting anti-lipoidal antibodies in serum or plasma. This assay is currently being used globally with great success. It offers laboratories a very cost-effective method to test for syphilis without requiring any other laboratory equipment.
This test is mostly performed for three reasons:
- as a screening method which is then confirmed with another treponema specific assay (TPPA etc.)
- To confirm a screening which has been performed with a treponema specific test (TPPA etc.)
- To follow up on treatment by performing titers (1:2, 1:4, 1:8 etc.) on samples during treatment.
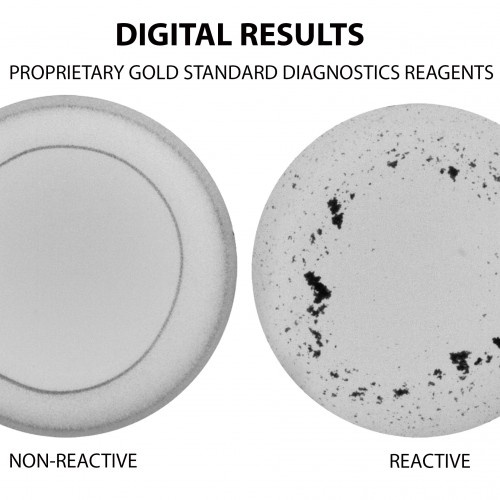
The RPR test is based on a reagent containing antigen coated particles. If antibodies to this antigen are present in a sample the particles will flocculate.
We are able to offer both the manual and an automated RPR test. The fully automated version was developed by Gold Standard Diagnostics (GSD), a company in the United States of America. They were the first company to develop an automated RPR test. The test is FDA and CE approved.

The AIX 1000 is able to process up to 192 samples in one run and can be connected to the LIS system for maximum traceability and ease-of-use.